What Causes Ice to Melt?

Overview
Overview
Keywords: temperature, ice, glaciers, melting, heat, cooling, global warming, climate change, scientific control
Subjects: science, physics, geography, social studies
Age group: 5-12 years old
Difficulty level: ● ○ ○ (easy)
![]()
This experiment is part of the unit "Climate Action" written by: Esra Aksoy (TR), Rebecca Mudde (GB), Rute Oliveira (PT), Anastasia Papakonstantinou (GR)
A solid substance melts, i.e. it changes from a solid to a liquid state, when it is heated. If we look at the smallest particles (e.g. molecules) that make up a substance, we can imagine it like this: The particles, which can initially move very little due to the interactions between them (solid), move faster and faster when heat energy is added.
With a sufficient supply of energy, the movement of the particles eventually becomes so great that the interactions between the particles are partially overcome and the particles can move freely. This means that we can observe that the substance melts and becomes liquid.
This experiment can introduce the students to the idea of global warming causing the polar ice caps to melt.
This project is aimed to be cross-curricular linking in with
- geography (recognising the various regions of the planet and their characteristics),
- physics (physical states of matter and changes of physical states),
- and social studies (understanding the impacts of climate change).
The experiment
Required materials
- 3 transparent glass containers (if using a heat source such as a heat lamp, do not use plastic containers as these may melt)
- ice cubes of the exact same size
- a thermometer
- a timer/stopwatch
- a heat source (e.g. infra-red lamp 175 W, terrarium heat lamp, a radiator, or the sun)
- a fridge or cool area
- a notepad and pencil
Experiment guide
This experiment can be performed by the teacher for the class, but depending on the materials available, age and autonomy level of the students, they can perform the experiment in small groups.
- Place ice cubes of the same size in three individual glass containers.
- Place the containers in three different places. One container next to a heat source, one container at room temperature as scientific control, and one container in a shaded or cool place like a fridge.
- Set a stopwatch to measure how long it takes for the ice cubes to melt.
- Record the results.
Questions for students
- What will cause the ice to melt?
- Why do the ice cubes melt more slowly when put in a cool place?
- Why does the heat source cause the ice to melt faster?
The first worksheet will introduce the children to the scientific method. They learn under which conditions ice melts and how these conditions affect how fast the ice melts. Students will understand the reasons for the melting but most importantly, they develop environmental empathy.
The worksheet tasks can be completed independently or in a group discussion.
It is available in two versions:
- Easier version A (for ages ca. 5-8) as docx and pdf
- More challenging version B (for ages ca. 9-12) as docx and pdf
The second worksheet introduces the children to the states of matter and the changes in the states of matter.
The experiment with the result
Everything around us, like the air we breathe, the water we drink, the food we eat, is made up of tiny, invisible building blocks called molecules. Even if we can't see them, these molecules are in constant motion and the higher the temperature around them, the more energy they have and the faster they move.
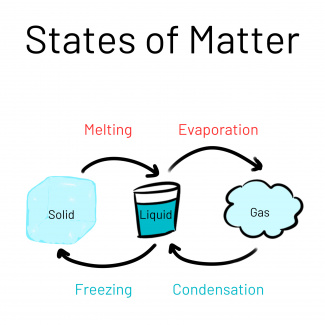
Matter, like water, can be in a solid, a liquid or a gaseous state. The physical state a substance is in depends on the interactions that the molecules (the small, invisible building blocks of all matter) have with each other. In the solid state, the interactions are the strongest, and in the gaseous state there are no more interactions between the particles.
When liquid water is placed in the freezer, the water molecules move less quickly, and their interactions become stronger. At below zero degrees Celsius, this leads to the transition of the aggregate state from liquid to solid (freezing). If the temperature is above zero degrees Celsius, the transition from the solid to the liquid state takes place - ice begins to melt (melting).
At the particle level, this means that the water molecules move faster and the interactions between the molecules are reduced. The higher the temperature, the faster the ice melts. For this reason, an ice cube melts faster on the outside and remains cold and solid for longer in the middle.
If the liquid water is heated further and the temperature reaches the boiling point of water, around 100 degrees Celsius (at normal pressure), the water particles no longer interact with each other as they move so quickly - water vapor is formed (evaporation). If the temperature falls below 100 °C, the water vapor condenses again (condensation).
How does this video link to sustainability?
This project aims to highlight the impact of global warming on the increased melting of the polar icecaps and the global consequences that this may cause. Students will gain understanding of how temperature can impact melting of ice and how increasing global temperatures can have an impact on all areas of sustainability.
Differentiated learning
Explore ideas how you can use this material in your class and adapt it to a group of various levels and learning styles.
Easier
- The identification game: Use cards with pictures of substances in different physical states. Ask the children to sort the cards into piles of solids, liquids and gases.
- Show the children samples of materials in different physical states and discuss each one, placing them in one of the categories (solid, liquid or gas).
More challenging
- Students can record their results in a graph or table.
- Students may draw a diagram of what is happening to the water molecules as the ice cube melts.
- Very strong or older students can discuss the question: What is happening to the molecules in the ice cube?
- To help children better understand how the agitation of particles affects their physical state, a simulation of the physical states of matter in phet can be used.
New vocabulary
New vocabulary can be introduced using the theme of the states of water.
- Liquid water: oceans, seas, rivers, lakes, groundwater
- Ice: glaciers, icebergs, polar ice caps
- Water vapour: steam
Water vapor is any gaseous water below boiling temperature, usually invisible.
Steam is hot, often pressurized water vapor at or above boiling temperature, which appears as a visible mist when it condenses in cooler air.
Examples of steam that children might easily observe:
boiling pot of water, hot shower, kettle whistle, hot food, ironing clothes
These words can be accompanied by images and displayed in the classroom.
Role play
Role play about the physical states of matter:
Begin with a brief description of the physical states of matter. Divide the children into three groups. Each group will represent one of the states of matter (solid, liquid, gaseous). Children should represent molecules of a substance and represent how they behave in the 3 physical states. They can represent how far away they are and how they move. The activity can be carried out in the school playground and a container can be drawn in chalk on the floor where the molecules of the substance, represented by the children, should be during the role play.
Children can investigate what other factors can affect the melting speed of ice:
Investigate whether the same mass of ice melts faster if it's in a large ice cube or crushed ice.
- Result: When ice is crushed, the pieces are smaller and there are more surfaces exposed to the surrounding temperature. This allows heat to transfer more quickly into the ice, causing it to melt faster.
Investigate whether the container the ice is placed in influences its melting speed, for example if the ice is in a plastic dish or on a metal surface.
- Result: Yes, the material of the container influences how quickly the ice melts. Ice will melt faster on a metal surface than on a plastic one because metal is a better conductor of heat.
Investigate whether the ice melts at the same temperature if the water contains salt.
- Result: When salt is added to ice, it lowers the freezing point of water. This means that salty ice will start to melt at a temperature lower than 0 °C, which is the melting point of pure ice.
Investigate whether it makes a difference to the melting speed of the ice if the container has a lid.
- Result: A container with a lid slows down the melting process of ice compared to an open container. This is because the lid helps to trap cold air around the ice, reducing the amount of warm air that can circulate and transfer heat to it.
Investigate whether the water level rises when ice in water and ice on land are melting.
- This question is explored in the experiment: How does the Melting of Sea Ice or Land Ice affect the Sea Level?
Career orientation
Which career options are linked to this experiment and how can you introduce them to your students?
With this experiment on the problem of melting ice, children do not only discover the impacts of climate change, but can also be inspired to consider future careers in environmental protection. This could be a good opportunity to spark interest in professions that seek to understand and find solutions to climate change, such as: environmental scientist, oceanographer, marine geologist and engineer.
Environmental scientist
An environmental scientist works as a researcher to understand climate change patterns and sea level impacts.
What is an environmental scientist?
Environmental scientists are nature detectives! They study the Earth and all living things on it to understand how they work together. Their job is to protect the environment and make sure it stays healthy for plants, animals, and people.
What does a day in the life of an environmental scientist look like?
In their day-to-day work, environmental scientists take for example samples of soil, water and air in different places, such as forests, beaches, rivers, seas or cities. They then take these samples to the laboratory to analyse them with various instruments, such as microscopes. Once the analyses have been carried out, it's time to sit down at a desk and analyse the results, writing reports to share their findings with other scientists and institutions that benefit from this knowledge.
What responsibilities do they have?
Environmental scientists have high responsibilities. Their scientific results are fundamental to find solutions to climate change. It is important that their findings are communicated to policy makers and the general public so that they can protect the environment and the climate. They are trying to ensure that we can all live on a healthy planet.
Oceanographer
An oceanographer studies the oceans, including changes in sea level and the effects of climate change on marine ecosystems.
What is an oceanographer?
Oceanographers explore the sea! They try to protect marine living beings by investigating the characteristics of the oceans. They also study how climate change is affecting ocean life, ocean currents or the acidity of the water, which in turn affects life in the ocean.
What does a day in the life of an oceanographer look like?
Oceanographers can for example start their day with a dive in the ocean. With special equipment, they collect samples of water, animals and plants, which they analyse when they are back in their laboratory. Oceanographers use various devices that analyse parameters such as water temperature, the amount of oxygen in the water, or the speed and direction of currents to predict the behaviour of the oceans.
What responsibilities do they have?
Oceanographers have a very high responsibility, ensuring that the oceans remain healthy. To achieve this, they publish their research to the community and promote sustainable fishing practices. Oceanographers also study the evolution of marine life and ecosystems over time.
Engineer
An engineer is a person who applies scientific knowledge and mathematics to develop solutions for technical problems.
What is an engineer?
An engineer is an expert at solving problems. Engineers use their scientific knowledge to create solutions that make our world better! They design levees, dams, bridges, buildings, robots, computers, and much more. To solve complex problems, engineers must be very creative to turn ideas into reality and solve complex challenges.
What does a day in the life of an engineer look like?
An engineer's day can for example start by brainstorming with colleagues to develop a project. An idea might arise for creating, improving or maintaining a machine or a product. Engineers can also spend their days building something, just like you are an engineer when you build a paper airplane or a parachute to play with. Climate change requires people to solve problems. Engineers might find solutions to this great challenge.
What responsibilities do they have?
There are many areas in engineering. Civil engineers build bridges, roads or houses. Mechanical engineers create machines and ensure that they work well. Marine engineers can design and manage coastal protection systems such as dikes and barriers. Regardless of their specialty, engineers make sure everything runs smoothly and safely.
Further ideas
We can use the theme of the physical states of water to link to many other themes explored in most primary school curricula, such as:
- the water cycle,
- the importance of water in our daily lives,
- the importance of saving water and ways of doing so,
- exploring the geography of water resources: identifying rivers, lakes, oceans and other sources of water on the map,
- exploring the properties of water, such as its density (floats, doesn't float),
- in Maths: measuring and/or calculating volumes.
States of matter in phet, PhETTM Interactive Simulations, University of Colorado, Boulder
(last accessed 18.11.2024)Sciencing: Making Science Fun for All Ages
(last accessed 18.11.2024)Melting Ice & Global Consequences, World Climate Research Programme (WCRP)
(last accessed 18.11.2024)Global ice loss is catching up to worst-case scenario predictions, World Economic Forum
(last accessed 18.11.2024)Career orientation videos:
Environmental scientist
Oceanographer
Engineer
(last accessed 29.10.2024)
This experiment is part of the unit "Climate Action" written by: Esra Aksoy (TR), Rebecca Mudde (GB), Rute Oliveira (PT), Anastasia Papakonstantinou (GR)
Share this page

